Urea Fertilizer Manufacturing: A Pillar of Modern Agriculture

Urea fertilizer is one of the most widely used nitrogen-based fertilizers in the world. Its high nitrogen content, cost-effectiveness, and versatility make it a staple in modern agricultural practices. With the growing demand for food due to population growth, urea fertilizer manufacturing has become a vital industry, ensuring farmers have access to the nutrients needed to boost crop yields and maintain soil health.
This article delves into the urea fertilizer manufacturing process, its significance in agriculture, market trends, and the environmental challenges associated with its production.
The Role of Urea Fertilizer in Agriculture
Urea, chemically known as carbamide (CO(NH₂)₂), is a nitrogen-rich compound that serves as an essential nutrient for plant growth. Nitrogen is a fundamental component of chlorophyll, the green pigment in plants responsible for photosynthesis. It also plays a crucial role in the synthesis of amino acids, proteins, and enzymes.
Urea fertilizer is particularly valued for its high nitrogen content, comprising 46% nitrogen by weight. This makes it one of the most concentrated nitrogenous fertilizers available, reducing transportation and application costs for farmers. Its versatility allows it to be used directly in the soil or in combination with other fertilizers, making it a critical input for cultivating a wide range of crops, from cereals and vegetables to fruits and cash crops.
The Urea Fertilizer Manufacturing Process
The production of urea fertilizer is a complex chemical process that begins with the synthesis of ammonia and carbon dioxide. These two key ingredients undergo a series of chemical reactions under high pressure and temperature to produce urea.
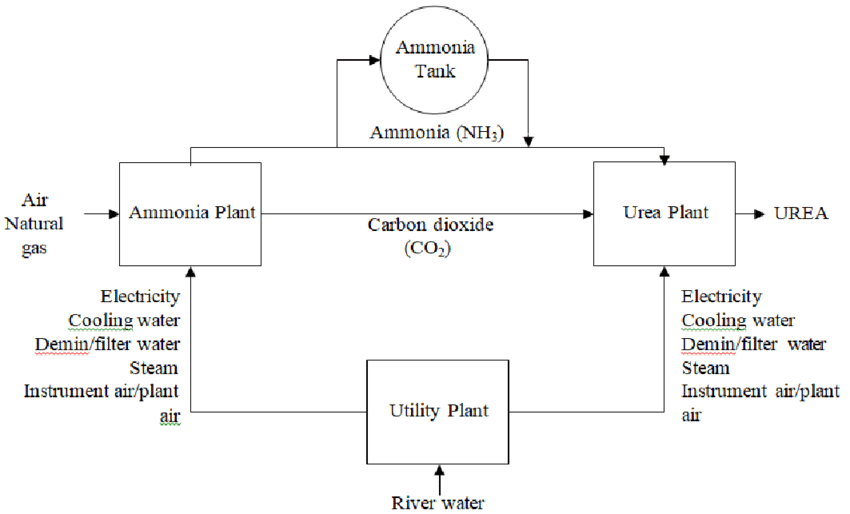
The process can be broadly divided into the following steps:
1. Ammonia Production
The manufacturing process starts with the production of ammonia, which serves as a primary raw material. Ammonia is typically produced through the Haber-Bosch process, which involves reacting nitrogen gas (N₂) from the air with hydrogen gas (H₂) under high pressure and temperature. Hydrogen is usually derived from natural gas or other hydrocarbons.
2. Urea Synthesis
In the next step, ammonia is reacted with carbon dioxide (CO₂) under high pressure to form ammonium carbamate, an intermediate compound. The ammonium carbamate is then dehydrated to produce urea and water. The chemical reaction can be represented as: 2NH3+CO2→NH2COONH4→(NH2)2CO+H2O2NH3+CO2→NH2COONH4→(NH2)2CO+H2O
3. Concentration and Prilling
The urea solution obtained from the synthesis process is concentrated by evaporating the water. The concentrated solution is then sprayed into a prilling tower or granulator, where it solidifies into granules or prills. These solid particles are dried, cooled, and screened to ensure uniform size and quality.
4. Packaging and Distribution
The finished urea is packed into bags or bulk containers for storage and distribution. It is then transported to agricultural markets or directly to farmers.
Applications of Urea Fertilizer
Urea fertilizer is widely used in agriculture due to its adaptability and effectiveness. It is applied directly to the soil as a solid or dissolved in water for foliar spraying. Urea is also a key ingredient in blended fertilizers, where it is combined with other nutrients to meet specific crop requirements.
In addition to its agricultural applications, urea is used in various industrial processes, such as in the production of resins, adhesives, and as a raw material for chemical synthesis.
Advantages of Urea Fertilizer
Urea fertilizer offers several advantages that have contributed to its widespread adoption. Its high nitrogen content means farmers need smaller quantities to achieve the desired nutrient levels in the soil. This reduces transportation and application costs, making it an economical choice for large-scale farming.
Furthermore, urea is highly soluble in water, allowing for efficient absorption by plants. Its versatility enables it to be used across different soil types and climatic conditions, enhancing its appeal to farmers worldwide. Additionally, urea is less prone to volatilization losses compared to other nitrogenous fertilizers, provided it is applied correctly and incorporated into the soil.
Challenges in Urea Fertilizer Manufacturing
Despite its benefits, the production and use of urea fertilizer present several challenges. The manufacturing process is energy-intensive, relying heavily on natural gas as a feedstock and energy source. This not only contributes to greenhouse gas emissions but also exposes manufacturers to fluctuations in natural gas prices.
Environmental concerns are another significant challenge. The excessive use of urea fertilizer can lead to nitrogen leaching, where nitrates seep into groundwater, posing risks to water quality and human health. Volatilization, where nitrogen is lost to the atmosphere as ammonia gas, can also occur if urea is improperly applied, reducing its effectiveness and contributing to air pollution.
Market Trends and Growth Drivers
The global urea fertilizer market is experiencing steady growth, driven by rising food demand and the need for improved agricultural productivity. Asia-Pacific is the largest market for urea, with countries like India and China leading in both production and consumption. These countries rely heavily on urea fertilizer to support their large agricultural sectors and ensure food security.
Technological advancements in manufacturing processes, such as energy-efficient reactors and improved catalysts, are helping reduce production costs and environmental impact. Additionally, the development of coated urea products, which release nitrogen slowly, is gaining traction as a sustainable alternative to traditional fertilizers.
Opportunities for Entrepreneurs in Urea Manufacturing
The urea fertilizer manufacturing industry offers numerous opportunities for entrepreneurs. With the global population projected to reach nearly 10 billion by 2050, the demand for food and, consequently, fertilizers, is expected to grow significantly. Entrepreneurs can capitalize on this trend by investing in modern, energy-efficient plants that reduce production costs and environmental impact.
Furthermore, the rising popularity of enhanced-efficiency fertilizers, such as coated urea and urease inhibitors, presents a niche market for innovation. These products help farmers maximize nitrogen use efficiency while minimizing environmental risks, creating a win-win scenario for both producers and consumers.
Sustainability in Urea Fertilizer Manufacturing
Sustainability is becoming a key focus in the fertilizer industry. Manufacturers are exploring ways to reduce the carbon footprint of urea production by adopting renewable energy sources and carbon capture technologies. For instance, some plants are experimenting with using green hydrogen, produced from water electrolysis using renewable electricity, as a replacement for natural gas-derived hydrogen in the Haber-Bosch process.
Additionally, practices such as precision farming and the use of slow-release fertilizers are helping reduce the environmental impact of urea application. By delivering nutrients directly to the root zone in controlled amounts, these methods improve nitrogen use efficiency and minimize losses.
Conclusion
Urea fertilizer manufacturing plays a pivotal role in modern agriculture, supporting food security and enhancing crop productivity worldwide. While the industry faces challenges such as energy consumption and environmental impact, advancements in technology and sustainability practices are paving the way for a more efficient and eco-friendly future.
For entrepreneurs and investors, the urea fertilizer sector presents significant opportunities to innovate and contribute to global agricultural development. By focusing on sustainability and adopting modern manufacturing practices, the industry can continue to thrive while addressing the environmental challenges of today.










